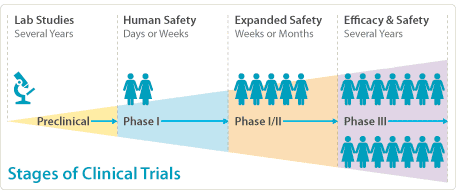
Do you know how a clinical trial gets started? When new drugs or devices need to be tested, trials are normally undertaken in 4 stages. If the first stage of trial is successful, the trial then qualifies for Phase two, and on to three and four. These later stages of research are conducted after approval by the regulatory body.
Before the first stage takes place, a pre-clinical phase involves tests on cells of a non-human variety that provide information about how effective it is, any problems of toxicity and how this impacts the drug organism or the device itself.
Phase I involves the testing of drugs on healthy human volunteers in a clinical trial, with a small dose on the first dose and then increased further but still remaining lower than a therapeutic dose. This stage used to be called ‘first-in-man’ but was replaced by a ‘first-in-human’ for better gender neutrality. This phase aims to test how much of the drug can be given to a person before any side effects become apparent. Find out about Adaptive Phase 1 Studies at a site like https://www.richmondpharmacology.com/specialist-services/adaptive-phase-i-studies
A small group of healthy volunteers will be invited to the clinic for clinical trials, so that they can be observed continually. Find out more about Adaptive Phase 1 Studies at a site like https://www.richmondpharmacology.com/specialist-services/adaptive-phase-i-studies
Phase II consists of testing new devices or drugs in patients for whom there will be a clinical benefit, checking for potential side effects and how effective it is. At this stage, the patients receive a safe therapy dose. After Phase I has established a dose level, this is the phase that determines whether this drug has real effects and how well it works. Some of the tests will involve Phase I and II combined for toxicity and efficacy.